Generics
Continually seeking safe, user-friendly dosing
Shiono Chemical carefully examines safety and effectiveness in our development and manufacture of generic pharmaceutical products, while complying with the various standards based on the pharmaceutical legislation in Japan. In addition, we are intent on enhancing user-friendliness such as controlling bitterness experienced and making dosage forms smaller by using the latest equipment and advanced techniques.
As a manufacturer and distributor of pharmaceuticals we develop highly safe and reliable generic products for patients, using our strength of superior information-integration capability that comes from being a trading company function, and making use of factory equipment with R&D functions.
A Wide Range of Dosage Forms
Maintaining diverse production lines in Japan and abroad
We have secured pharmaceutical production bases around the globe, in partnership with other pharmaceutical factories in Japan and abroad, in order to achieve a reliable supply of pharmaceutical products.
We offer a wide range of generic pharmaceutical dosage forms, using inventiveness also with container and packaging material, aiming to improve patients’ QOL.
-

Injection(plastic ampoule)
-
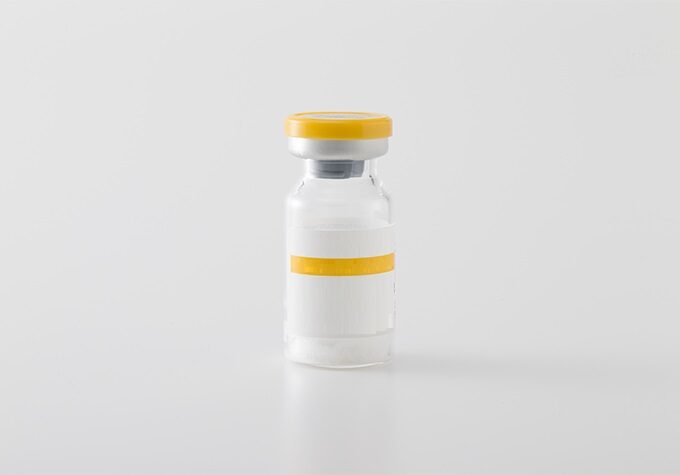
Injection(freeze-dried)
-

Injection(powder filling)
-

Injection
-
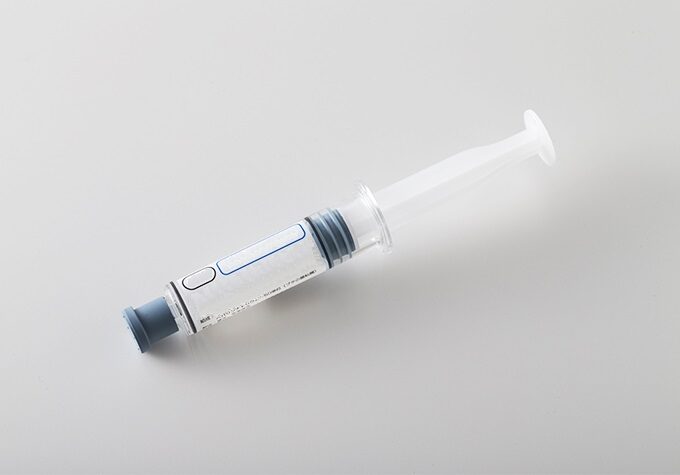
Injection(prefilled syringe)
-
Tablets
-
Orally disintegrating tablets
-

Special Packages for patient-friendly use
-
Capsules
-

Granules(fine granules)
-

Dry syrup
-

Ophthalmic solution
-

Tapes
-

Cream
-
Gel
-

Tapes(systemic action type)
-

Injection(bag for intravenous drip infusion)
-
Suppositories for rectal application
Takasaki Factory
Implementing quality control and quality assurance based on international standards
Shiono Chemical implements quality testing, quality control and quality assurance for generic pharmaceuticals at our international standard compliant factory.
The factory is organized with a thoroughgoing management system based on the large amounts of data already accumulated and careful planning, and is capable of not only generic pharmaceutical R&D, but also assessment of APIs and intermediates, and planning for and implementing approval application examinations.